Find Molarity Of Unknown Acid
An online titration calculator will help you to identify the unlike properties of a solution during an experiment. The titration molarity reckoner does the volumetric analysis titration calculations to calculate the titratable acidity.
What is Titration?
In chemistry, titration is a qualitative analysis technique, which can be used to compute the concentration of a specific analyte in a mixture. Titration is a vital technique in the field of belittling chemistry, sometimes called volumetric analysis.
During the titration process, a titrant/titrator is produced, which is a standard solution whose volume and concentration are specified. The titrant will react with the analyte until information technology reaches the endpoint or equivalence point, at which the analyte concentration tin can exist determined by measuring the amount of titrant used.
Types of Chemical Analysis:
Titration is a stoichiometric concept used to determine the unknown concentration of a solution. The field of chemical analysis can exist divided into two chief types.
Qualitative analysis: Where one determines the composition of a compound i.e. to determine which costless radicals are present in the salt.
Quantitative analysis: When the involvement is based on the concentration of an unknown solution.
However, an online Chemical Equation Balancer Calculator provides a balanced equation, structure, and equilibrium abiding with chemical names and formulas.
Acid-Base Titration:
According to the degree of dissociation, acids can be divided into strong acids and weak acids to form H + ions when they are dissolved in water. When using a potent base to titrate an acid solution of a known concentration, the acrid concentration can exist calculated, considering the fact that the neutralization reaction is complete. For the same reason, only stiff bases are used in the titration procedure, so in this case, acidic solutions are titrated, and strong bases are titrants or standard solutions.
Process of Acid-Base Titration?
· Take the required volume of brine/base of known concentration from the pipette and transfer it to the burette.
· Acid of unknown concentration is put in the burette and immune to interact with the base of operations drop by drop.
· The indicator that determines the endpoint is also placed in the titration bottle.
· At the stop of the reaction, the colour of the solution in the titration flask changes due to the indicator.
· The indicator used for this can exist phenolphthalein, which is pink in alkaline solutions and colorless in acidic and neutral solutions.
Therefore, the endpoint is determined when the pink solution becomes colorless. However, y'all tin can discover the volume, molarity, and moles of acid and base by titration calculator that are important for Acrid base titration.
How to Calculate Titrations?
When a base of operations or acid is dissolved in water, its H⁺ or OH– ions volition dissociate, which volition change the natural self-ionization balance of water:
2H₂O ⇌ OH⁻ + H₃O⁺
It will go more stiff acrid strong base titration solution. At pH 7, the concentration of H₃O⁺ ion and OH⁻ ion is i:i (titration equivalence point).
For titration, we commonly apply a solution of known volume but unknown molarity (analyte), to which a colored indicator (such as phenolphthalein) is added. When the ratio of 1:1 is reached (determined by the titration bend), the indicator will change color. By adding known molarity of acid or base (titrant) and measuring the amount required to upshot this alter, the titration calculator tin can calculate the molarity of the unknown value using the post-obit weak base strong acid titration formula:
nH * Vx * Mx = nOH * Vy * My
where:
nH = number of H+ ions contributed
Mx = molarity of the acid,
Vx = volume of the acrid,
nOH = number of OH- ions
My = molarity of base of operations, and
Vy = book of the base of operations.
However, a free Theoretical Yield Calculator is used for chemic reactions that compute the theoretical yield according to the theoretical yield formula.
Instance:
Dissolve 2.0 one thousand of the unknown monobasic acid sample in 100 ml of water. A 20 ml portion of this solution requires 15 ml of 0.12 Yard NaOH solution to reach the titrations equivalence signal. If the molecular mass of the acrid is 122 g/mol, determine the purity (%) of the acrid.
Solution:
The titration calculations for NaOH:
For 20 ml acrid solution: 15 ml 0.12 mol NaOH required
And then, the number of base equivalents = 12 × 15 = ane.8 × 10 -three equivalent
So, in 20 ml of acidic solution 1.80 x ten-3 equivalent of acids
Therefore x = ix × ten-three equivalent, because information technology is a monobasic acid, the mass of the titration equation of the acrid is the same.
In a 2 one thousand sample:
Acid mass = 9 × 10-3 × 122 = 1.098 g
% Purity = 1098/two × 100 = 54.9%
Tabular array of common bases and Acids and Strengths:
Base:
| Formula | Proper noun | Strength |
| NaOH | Sodium hydroxide | Strong |
| KOH | Potassium hydroxide | Strong |
| Ca(OH)ii | Calcium hydroxide | Strong |
| Ba(OH)2 | Barium hydroxide | Stiff |
| NH3 | Ammonia | Weak |
| CH3NH2 | Methylamine | Weak |
| C5H5N | Pyridine | Weak |
Acid:
| Formula | Name | Strength |
| HCl | Hydrochloric acid | Stiff |
| HNO3 | Nitric acrid | Strong |
| H2SO4 | Sulfuric acrid | Strong |
| HBr | Hydrobromic acid | Stiff |
| Howdy | Hydroiodic acrid | Stiff |
| HClO4 | Perchloric acid | Potent |
| HClO3 | Chloric acid | Strong |
| HCOOH | Formic acrid | Weak |
| CH3COOH | Acerb acid | Weak |
| C6H5COOH | Benzoic acid | Weak |
| HF | Hydrofluoric acrid | Weak |
| HNO2 | Nitrous acid | Weak |
| H3PO4 | Phosphoric acid | Weak |
Lab Equipment For Titration:
The experiment will be successful only if the correct equipment is used carefully. You must accept sufficient cognition of the equipment and its utilise. You should besides be familiar with some laboratory equipment during titration such every bit burettes and pipettes. They have some similarities but don't misfile them with each other, as this will pb to incorrect results.
Burette- is a long cylindrical drinking glass tube used to bring a solution of known concentration into a solution of unknown concentration. There is a stopcock or tap at the bottom to pour the solution into the flask, and in that location is a hole at the top. It has volume markings forth with the liquid level measurement.
Pipette- is a glass tube used to measure very pocket-size amounts of liquid. It is a long tube with a bulge in the middle. It is used to measure out the corporeality of solutions or titrants of unknown concentration. Information technology is smaller than a burette.
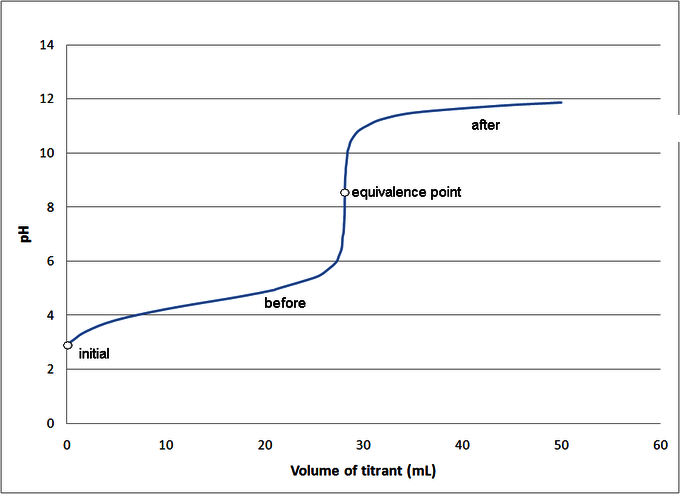
Estimating the Equivalence Signal's pH:
The pH value of the solution obtained at the equivalence bespeak depends on the relative concentration of acid and base. You tin estimate the pH value of the equivalence indicate according to the post-obit rule:
- Strong acid reacts with weak base to form an acidic solution (pH < seven).
- Strong acid reacts with strong base to form an acidic solution (pH = 7).
- Weak acid reacts with strong base to course an acidic solution (pH > 7).
When a weak acid reacts with a weak base of operations, the equivalence point solution is alkaline/base if the base is strong, and acidic if the acid is strong; if the two concentrations are the same, the equivalent pH value is neutral. However, weak acids cannot ordinarily exist titrated with weak bases considering the color change is short-lived and therefore difficult to detect.
How does Titration Calculator Works?
The titration concentration computer computes the concentrations and volume of acid base solution by following these guidelines:
Input:
· Get-go, choose an choice from the drop-down listing as you want to summate the titration.
· Now, substitute the values in the fields according to your selection.
· Hit the summate titration button.
Output:
The titration estimator compute:
· Molarity and book of the acid
· Molarity and volume of the base
· Moles of H+ and OH- Contributed
FAQ:
What are the main types of titration?
The chief types of Titration are:
· Acid-base Titrations
· Redox Titrations
· Precipitation Titrations
· Complexometric Titrations
What is the purpose of acrid-base titration?
The main purpose of acid base titration is to determine an unknown concentration of acid or base of operations in a solution past neutralizing it with a known concentration of base or acid.
What are acid-base indicators?
A substance that changes color when the pH of the surrounding changes is chosen an acid-base indicator. They are as well called pH indicators.
What is cease point in titration?
In the titration process, the indicator indicates the point at which the amount of reactant required to complete the reaction has been added to the solution.
Conclusion:
Employ this online titration calculator for acid and base titration calculations that helps you to identify the properties of a solution during the chemical experiments. Titration is the slow addition of i solution of known forcefulness to some other solution of unknown forcefulness until neutralization. Therefore, knowing the book value of the two different acid-base of operations solutions and the intensity value of each acid/base volition atomic number 82 to the unknown solution'southward intensity value of the unknown solution. And so, utilise our gratuitous online calculator to perform titration calculations chop-chop.
Reference:
From the source of Wikipedia: Volumetric analysis, Preparation techniques, Titration curves, Types of titrations, Redox, Gas phase, Complexometric, Zeta potential, Analysis.
From the source of LibreText: Acid-Base Titrations, Complexation Titration, Titration of a Weak Acid with a Strong Base, Titration of a Weak Base with a Strong Acid.
From the source of Lumen Learning: Setting up an Acid-Base, Materials for a Titration Procedure, Equivalence Indicate Indicators, Estimating the Equivalence Point's pH.
Find Molarity Of Unknown Acid,
Source: https://calculator-online.net/titration-calculator/
Posted by: marchfaryinly.blogspot.com


0 Response to "Find Molarity Of Unknown Acid"
Post a Comment